A research team from Nanyang Technological University, Singapore (NTU) and the National University Health System (NUHS) has found a new way to tackle the growing obesity epidemic around the world.
The team,led by Professor Louis Phee, NTU Dean of Engineering and Professor Lawrence Ho, a clinician-innovator at NUHS, has designed a pill, named Endopil.
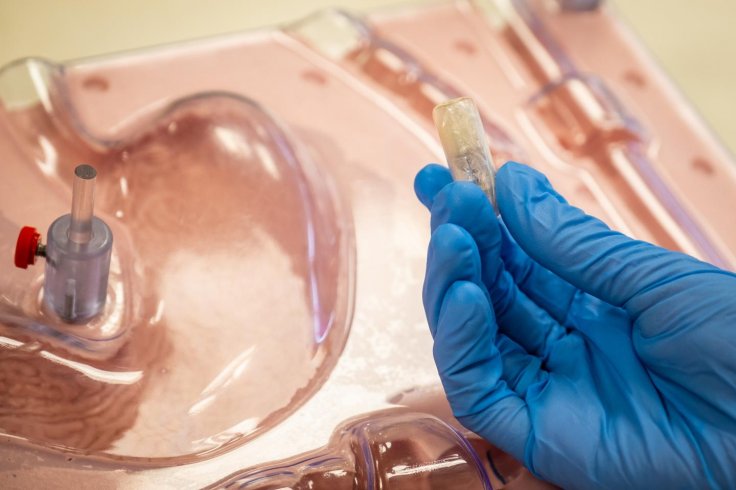
The EndoPil is a prototype capsule containing a balloon. It has to be taken with a glass of water, which, under magnetic stimulation, causes a reaction between a harmless acid and salt stored in the capsule, which produces carbon dioxide to fill the balloon.
Currently, a similar technique in use is the Endoscopic Intragastric balloon, a procedure where 1 to 3 inflated silicon balloons are placed in the stomach for 6 months, making less room for food. It results in less intake of food, leading to loss of weight.
But its major drawback, according to Ramsey Health UK, is bleeding or perforation in case of injury during the balloon insertion or removal, gastric discomfort, nausea and vomiting during the first few days following balloon placement. A sense of heaviness in the abdomen, abdominal or back pain, gastro-oesophageal reflux (where stomach acid leaks out of the stomach) or indigestion are the other side effects. Although rare, leakage or deflation of the balloon could also occur, according to experts.
In view of these risks, this new method promises to keep these drawbacks at bay with its simplicity of administration. "All you would need is a glass of water to help it go down and a magnet to activate it. We are now trying to reduce the size of the prototype, and improve it with a natural decompression mechanism. We anticipate that such features will help the capsule gain widespread acceptance and benefit patients with obesity and metabolic diseases," said Professor Louis Phee of NTU.
Co-lead Prof. Lawrence Ho explained that EndoPil's compact size and simple activation using an external hand-held magnet could pave the way for an alternative that could be administered easily by doctors even within the outpatient department (OPD) and in primary clinics. "This could translate to no hospital stay, and cost-saving to the patients and health system," he said.
The EndoPil will potentially erase the need for tubes to be inserted through the nasal, oral and oesophagal openings. It tackles the issue of discomfort and the vomiting sensation by allowing for shorter treatments since the balloon stays for just a month, and hence, the stomach does not adapt structurally to it.
The team is working on a bio-degradable structure for the balloon, and for it to deflate by an external magnet.
The original balloon inflating mechanism was granted a US patent in 2016, according to the scientific journal PLOS ONE. The Singapore team said it has filed for an entirely new US patent for their latest innovation. The team hopes to conduct another round of human trials for at least one year to ensure that the prototype can be naturally decompressed and expelled by the body, before testing the capsule on humans for its efficiency in treatment.









