French drugmaker Sanofi is reportedly planning to enroll thousands for its experimental coronavirus vaccine trials. Sanofi is working on two potential Covod-19 vaccines and said that it is exploring several manufacturing options so that it can meet the global demand of either of the vaccines succeed.
Drugmakers across the world have been working towards developing a Covid-19 vaccine, with many pharmaceutical giants joining forces to ramp up research and development and manufacturing process. Sanofi had earlier said that it could produce 600 million doses of coronavirus vaccine by 2021 if clinical tests succeed.
Sanofi ramps up Covid-19 vaccine trial

Sonofi is reportedly planning to enroll thousands of subjects globally for trials of a coronavirus vaccine candidate it has developed with GlaxoSmithKline (GSK). Following the coronavirus outbreak, Sanofi joined forces with GSK to develop a vaccine for the deadly virus. The vaccine candidate is in its Phase 1 trial.
While most Phase 1 trials involve a smaller number of volunteers, Sanofi is going for higher numbers in a bid to secure stronger data to support its vaccine development. According to Reuters, the company is also exploring manufacturing options that also includes fresh collaborations with drugmakers to ensure that it can meet the global demand if it succeeds in developing a vaccine.
Sanofi presently is working on two Coviod-19 vaccine candidates and has already started discussing advance purchases with several countries. Sanofi is hopeful that a vaccine for Covid-19, which has killed more than 257,000 globally, will be ready by 2021.
Under the agreement with GSK, Sanofi is bringing in a protein antigen, a molecule designed to trigger an immune response in the body, which the drugmaker uses for its influenza vaccine. GSK will be contributing on its approved adjuvants, which aid in giving a boost to the immune response to produce antibodies and longer-lasting immunity.
Sanofi confident of a vaccine by 2021
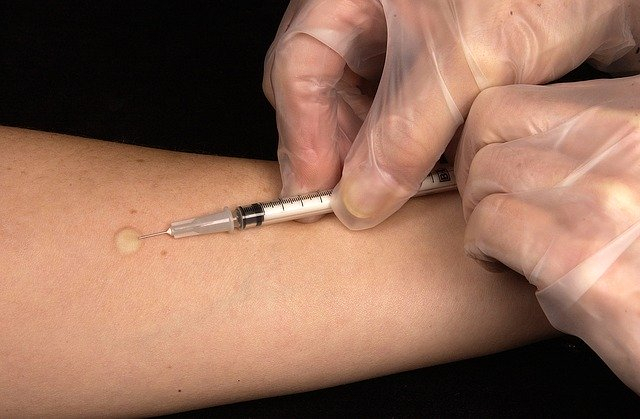
More than a 100 Covid-19 vaccine candidates are in development stage, with only 10 managing to reach the clinical trial stage so far, according to the World Health Organization. Sanofi is hopeful that the early-stage trials would start by Sepetmber. The company also is confident of coming up with a vaccine by 2021.
However, production and distribution remains a concern. Sanofi, last month, had said that it would be able to produce up to 600 million doses of vaccine next year. Sanofi CEO Paul Hudson had said, "We believe we're one of the few companies who will be able to make a vaccine at a huge scale."
There are currently no drugs or therapies to treat Covid-19 and drugmakers across the world are speeding up development to come up with a vaccine, which most say will take at least 12 to 18 months. Sanofi's project with GSK has got financial assistance from the Biomedical Advanced Research and Development Authority (BARDA) of the US Health Department.
Sanofi is separately working with Translate Bio on another vaccine candidate based on messenger RNA technology (mRNA). The vaccine candidate is similar to experimental vaccines being developed by Pfizer in partnership with BioNTech and another one by Moderna, which is being developed in partnership with the US government.








