Japan's top research institutes in collaboration with pharma companies have decided to explore the possibility of using Nafamostat inhalation formulation for the treatment of novel corona virus infection or COVID-19.
The University of Tokyo announced these new findings on March 18, 2020 and the new project will accelerate the R&D to make it available by March 2021. Nafamostat is an injectable used as a treatment for acute pancreatitis and disseminated intravascular coagulation for many years in Japan. The new joint project seeks to explore its possible extension to treat Covid-19 infection.
Colaboration for Covid-19 Inhaler
The University of Tokyo, RIKEN, which is Japan's largest scientific research institute with 3,000 scientists on seven campuses, Nichi-Iko Pharmaceutical company, and Daiichi Sankyo Company have reached a basic agreement on collaborative research and development (R&D) on a Nafamostat inhalation formulation for the treatment of novel corona virus infection.
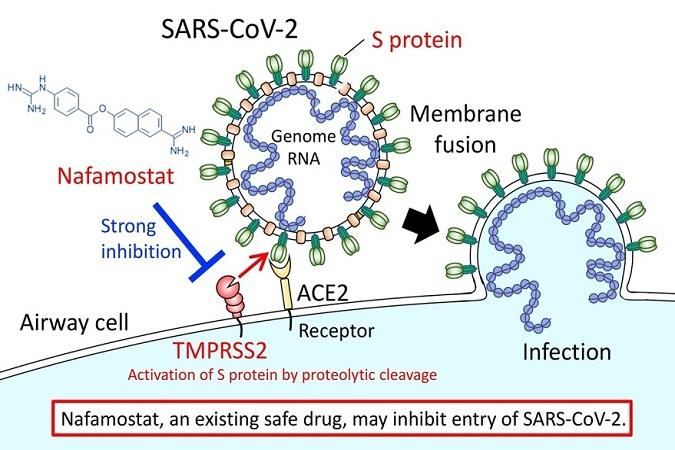
In the first stage of infection by the causative virus of SARS-CoV-2, the outer envelope of the virus fuses with the host cell surface membrane. Prof. Junichiro Inoue of the University of Tokyo and others have discovered that by preventing this fusion, Nafamostat could efficiently inhibit the entry of the virus effectively.
RIKEN has been focusing on drug discovery to optimize the medical findings from basic research for use in the drug discovery process at pharmaceutical companies, and in clinical trials as a bridge to companies and medical institutions. For this research, RIKEN will contribute in terms of using its multidisciplinary advanced technologies, said the statement.
Nichi-Iko, which holds rights to market FUTHAN (generic name: nafamostat mesilate), will provide data collected on the product over many years as well as supply the API for the research collaboration. Daiichi Sankyo will carry out research on the Nafamostat inhalation formulation using technology gained in the development of its anti-influenza virus agent, Inavir.
Non-clinical studies are scheduled to begin in July this year and after consultation with authorities, it will enter the clinical trial possibly by March 2021. Professor Inoue and Assistant Professor Mizuki Yamamoto of the Research Center for Asian Infectious Diseases of the Institute of Medical Science, the University of Tokyo, have identified Nafamostat as a strong candidate to fight COVID-19.
"Considering that SARS-CoV-2 infection is already spreading worldwide, drug repurposing, which searches for therapeutics among existing drugs with established safety records, seems to be extremely worthwhile," Inoue said.
Future Potential of Nafamostat
The genomic RNA of coronaviruses such as SARS-CoV-2 is surrounded by an envelope composed of a lipid bilayer and envelope proteins. SARS-CoV-2 initiates human cell entry after the Spike protein (S protein) present on the envelope binds to a cell membrane receptor ACE2. As ACE2 and TMPRSS2 are essential in airway cells for SARS-CoV-2 infection, they are targeting the entry level of the coronavirus.
Reported in 2016, Nafamostat effectively inhibits MERS-CoV S protein-initiated membrane fusion and it led the scientists to recommend that Nafamostat could be effective at inhibiting MERS-CoV infection. Since the new SARS-CoV-2 has similar traits, a similar experiment was performed using Nafamostat, which significantly inhibited SARS-CoV-2 S protein-initiated fusion.
Nafamostat is administered clinically by intravenous infusion and the blood concentration of it would exceed the concentration needed experimentally to inhibit membrane fusion via the SARS-CoV-2 S protein. Hence, the researchers hope that Nafamostat will prevent SARS-CoV-2 from entering human cells.









