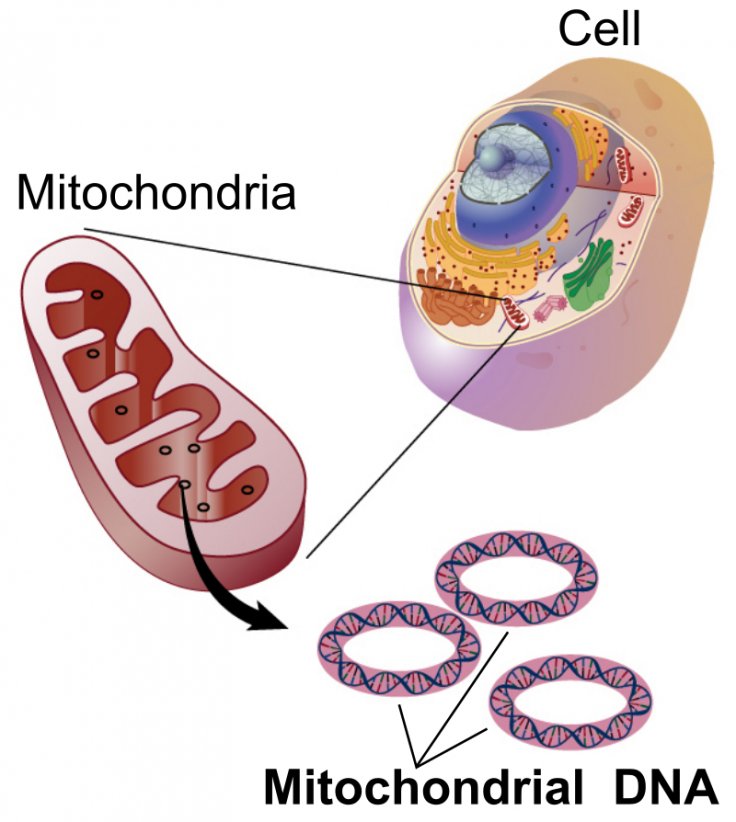
Clustered Regularly Interspaced Short Palindromic Repeats or CRISPR, is the most popular yet controversial gene-editing method available. However, scientists have now found a new method to edit mitochondrial DNA precisely by exploiting interbacterial toxins, that can enable the study of mitochondrial diseases and potentially cure them.
The technique, which is not CRISPR based, has been developed through the collaboration between Howard Hughes Medical Institute (HHMI), US researchers — Dr. Joseph Mougous, of the University of Washington, Dr. David Liu, of Harvard University and the Broad Institute and Dr. Vamsi Mootha, an HHMI investigator at Massachusetts General Hospital and the Broad Institute. The gene-editing tool will use interbacterial toxins—which are produced by bacteria to fight each other—to study the basic mitochondrial biology apart from diseases, albeit, in animals for now.
"This is a transformative technology for my field. It will now be possible to create mouse models of mitochondrial DNA (mtDNA) disease—this has been super difficult until now," said co-researcher Mootha.
CRISPR Method Not Enough
The research, which was published in the journal Nature on July 8, elaborated on the need to introduce modifications into mtDNA. In the existing CRISPR method, a targeted RNA takes the Cas9 enzyme to a genome and make changes in the DNA. But it is not helpful in modifying mitochondria as some of its cells cannot be accessed through the method.
Hence, scientists had to rely on erasing an organelle's DNA to get rid of mutations in mitochondrial diseases. Nevertheless, the risks were that it destroyed entire genes and not just a single mutation. The new method does not rely on the RNA to direct the Cas9 enzyme. Thus, it is able to get into the mitochondria and make single nucleotide changes on the double-stranded DNA.
The new study focused on harnessing the power of such naturally occurring antibacterial toxins to target genetic mutations. Dr. Liu's student Beverly Mok suggested a method to eliminate the toxicity, dividing the deaminase, an enzyme, into two harmless halves. However, since one harmless half would not be a gene editor, they fused each half of the proteins that could reach specific regions of mitochondrial DNA.
"An alternative to the targeted destruction of mtDNA through double-strand breaks is precision genome editing, a capability that—to the best of our knowledge—has not been previously reported for mtDNA," said Dr. David Liu of Harvard University and the Broad Institute, co-author of the paper.
How Can This New Method Help?
At present, there is no cure for mitochondrial diseases that mostly manifest at birth. Common mitochondrial diseases such as Mitochondrial myopathy, Diabetes mellitus and deafness (DAD), mitochondrial DNA depletion syndrome (MDDS), MELAS syndrome can only be managed. They are the result of single point mutations in multiple genes that attack the muscles and the brain.
While the new technique will take years to cure a human, scientists managed to repair 20-40 percent of the targeted genes in human cells within 3-6 days. Though the new technique looks promising, it still requires further research. Compared to a maximum 40 percent accuracy, the target is now to improve to 80 percent and above. For now, it will help scientists generate animal models to study the mitochondrial abnormalities in detail.
"The ability to precisely install or correct pathogenic mutations could accelerate the modeling of diseases caused by mtDNA mutations, facilitate pre-clinical drug candidate testing, and potentially enable therapeutic approaches that directly correct pathogenic mtDNA mutations," the authors said. "Bacterial genomes contain various uncharacterized deaminases (enzymes), raising the possibility that some may possess unique activities that enable new genome-editing capabilities."









