For millions of people suffering from different forms of cancer, immunotherapies have granted a second shot at a healthier life. The approach, however, deals with triggering the adaptive immune system (T-cells and B-cells) of the body and its effectiveness varies from person to person. A new study has demonstrated that the innate immune system—a more primal immune system of the body—can be trained or 'primed' to destroy cancer cells.
The study by an international team of researchers has discovered that when the innate immune system is "trained" with β-glucan—a substance derived from fungus—triggered the production of innate immune cells, particularly neutrophils, in animal models after being primed to attack tumor cells. The findings can help relook approaches to immunotherapy and improve cancer treatment.
"The focus in immunotherapy is placed on adaptive immunity, like checkpoint inhibitors inhibit the interaction between cancer cells and T cells. The innate immune cells, or myeloid cells, have not been considered so important. Yet our work suggests the myeloid cells can play a critical role in regulating tumor behavior," said Dr. George Hajishengallis, co-senior author of the study, in a statement.
Turning to Innate Immune System for Cancer Relief
The innate immune system is an emergency nonspecific immune response that the body launches against antigens invading the body till antibodies are generated, and lymphocytes known as T-lymphocytes or killer T-cells are dispatched to fight the pathogens. On the other hand, the adaptive immune system relies on the "immunological memory" created during the encounter with a pathogen and developing improved immune responses against in the event of another attack in the future.
A previous study led by Dr. Hajishengallis found that trained immunity provokes through the exposure to the β-glucan compound was able to improve immune recovery following chemotherapy in mice. Through the study, the researchers had also illustrated that the "memory" of the innate immune system was in the bone marrow—within the hematopoetic stem cells that give rise to myeloid cells such as macrophages, monocytes, and neutrophils.
Through the current study, which builds on the previous paper, the team sought to acquire deeper insights into the mechanism that was involved in the encoding process of the "memory". "The fact that β-glucan helps you fight tumors doesn't necessarily mean it was through trained immunity," stated Dr. Hajishengallis.
Transferring Trained Properties to Vulnerable Mice

In order to confirm the link between memory of myeloid precursors and trained innate immune response, the authors extracted neutrophils from mice that had been subjected to innate immune training through exposure to β-glucan. These neutrophils, along with cells develop into melanoma tumors, were transferred to mice that had not been exposed to β-glucan. The growth of tumors in these mice was found to be considerably suppressed.
Next, the team aimed to find more evidence to support the link. First, they conducted bone marrow transplants. They irradiated mice and effectively eliminated their bone marrow. Then, these mice received bone marrow transplants from mice whose immune systems were "trained".
The experiment was based on the hypothesis that the progenitor of neutrophils in the bone marrow of donor mice would be transferred to the recipient mice and lead to the production of neutrophils with tumor-destroying capabilities
When faced with cancer later, the mice that had received bone marrow transplants from trained mice were able to counter tumor cells more efficiently than untrained mice. "This is innate immune memory at work," highlighted Dr. Triantafyllos Chavakis, co-senior author of the study.
Genetic Changes Priming Tumor-fighting Abilities
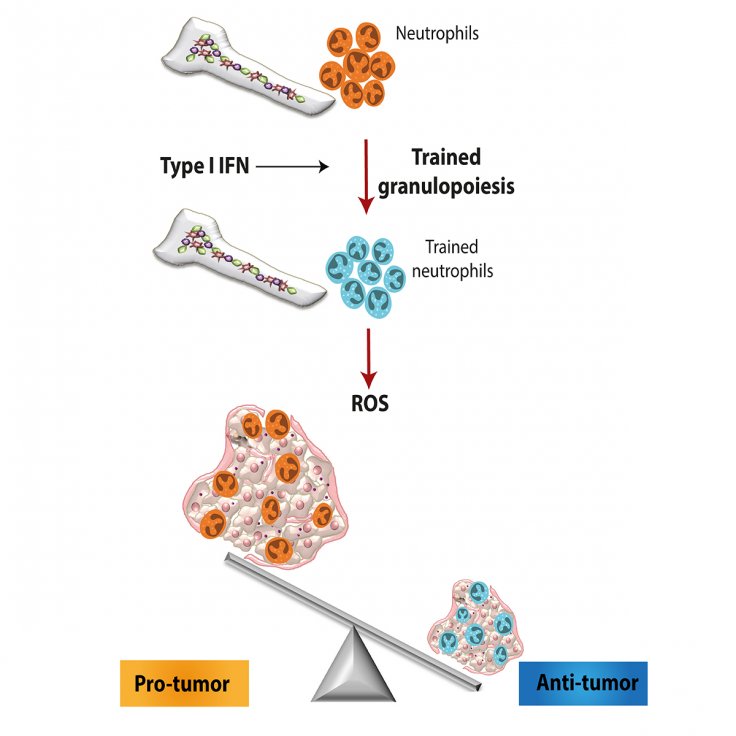
According to the authors, the anti-tumor was a likely result of the trained neutrophils producing increased levels of reactive oxygen species (ROS) in comparison to untrained neutrophils. While ROS is harmful in certain health contexts, in the case of cancer, they can be beneficial as they work towards the killing of tumors.
Closer evaluation of the myeloid precursors found in the bone marrow of the trained mice revealed crucial genetic changes that promoted the development of neutrophils, particularly one known to exhibit anti-tumor activity—tumor-associated neutrophils type I (TAN1). The authors found that these alterations initiated by innate immune training led to the epigenetic rewiring of bone marrow precursor cells.
These changes made specific genes more accessible to being transcribed. Additionally, they also likely caused the Type I interferon signaling pathway to function as the moderator of the innate immune training. "This is a breakthrough concept that can be therapeutically exploited for cancer immunotherapy in humans, specifically by transferring neutrophils from β-glucan-trained donors to cancer patients who would be recipients," concluded Dr. Hajishengallis.









