In a phase one clinical trial of China's vaccine, it was found to be safe and well-tolerated, while generating an immune response against the novel coronavirus infection in humans. An open-label trial of 108 healthy adults showed good results after 28 days. However this is not the final trials and the overall results would be evaluated in six months.
These results, published in The Lancet Friday, mark mark a milestone, according to Prof. Wei Chen who heads the study at the Beijing Institute of Biotechnology in China. He said that a single dose of the "new adenovirus type 5 vectored COVID-19 (Ad5-nCoV) vaccine produces virus-specific antibodies and T cells in 14 days, making it a potential candidate for further investigation."
More Research Needed
However, he added that these results be interpreted with caution, as there were unprecedented challenges in developing an effective coronavirus vaccine. He said that mere ability to trigger immune response doesn't indicate that a vaccine would protect us from COVID-19.
"We are still a long way from this vaccine being available to all," he said. The new Ad5 vectored COVID-19 vaccine is one among more than 100 vaccine candidates in development worldwide.
How It Works?
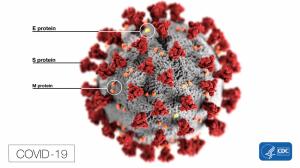
This is its first test in humans. The researchers used weakened common cold virus (adenovirus - does not cause a disease, but can get into a cell) that can deliver a genetic material in cells for the SARS-CoV-2 spike protein. These cells travel to the lymph nodes creating antibodies as an immune response which recognizes spike protein and fights the novel coronavirus.
The primary aim of the trial was safety and the vaccine's ability to give an immune response. The Ad5-nCoV vaccine was administered to 108 healthy adult volunteers aged between 18 and 60, in three dosages.
An ideal vaccine is expected to generate both antibody and T cells that defend the body from the virus. The one in discussion had done both, said researchers.
Reactions
The most adverse reaction was only pain in the injection site in 54 percent of the volunteers, while 46 percent got fever, fatigue for 44 percent, headache for 39 percent, and muscle pain for 17 percent. Most adverse reactions were only mild or moderate in severity and not serious
However, these reactions persisted for less than 48 hours. This means the vaccine was well tolerated at all doses within 28 days of vaccination.
In almost two weeks after vaccination among all dosages triggered some level of immune response by forming antibodies, some had detectable neutralising antibodies against the coronavirus. In 28 days most of them had a four-fold increase in binding antibodies (they only bind to the coronavirus and doesn not necessarily attack it)
Ad5-nCoV vaccine stimulated a fast T cell response among most of the volunteers in proportion to the dosages they received which peaked after 14 days of vaccination
Phase 2 Trials Started
The randomised, double-blinded, placebo-controlled phase 2 trial this vaccine is started in Chins's Wuhan to find if the results replicate with 500 healthy adult volunteers including participants aged more than 60 for the first time
However, pre-existing adenovirus (Ad5) immunity "may also have a negative impact on the persistence of the vaccine-elicited immune responses," says Professor Feng-Cai Zhu from Jiangsu Provincial Center for Disease Control and Prevention in China who also led this study.









