The WHO's war cry against the novel coronavirus pandemic was "test, test, test." Such is the importance of extensive testing in a rapidly worsening global health crisis where time is not money but life. In such a scenario, accurate and quick testing methods are essential for tackling the spread of COVID-19. Providing hope in this regard, researchers from China have developed a test that can detect antibodies against the SARS-CoV-2 virus in just 10 minutes.
The new test is a result of the collaboration between scientists from several institutions in Guangzhou, China, and builds on a testing technique known as a lateral flow immunoassay (LFA). An example of such a test is a home pregnancy test. It is based on the detection of the antibody, Immunoglobulin G (IgG), produced by the body to counter the coronavirus.
Expressing confidence over the new testing technique, the authors wrote in the study, "We expect this assay to be highly useful for helping to contain the COVID-19 outbreak by allowing timely diagnosis through early detection of SARS-CoV-2."
How does the test work?
LFA is a diagnostic device relied on to confirm the absence or presence of a target component or chemical substance such as contaminants in food or water supplies, and biomarkers such as antibodies or pathogens in animals or humans, among others.
For the new test, a viral coat protein was attached to a particular region on a strip of nitrocellulose. Following this, human serum was added. The serum was allowed to flow end to end on the strip. IgG antibody against the viral protein bound to specific regions on the strip. Using fluorescently labelled mouse antibody, the scientists detected the anti- SARS-CoV-2 IgG present in the serum. Unlike regular LFAs where the results can be observed with the naked eye, the sensitivity of the fluorescence-based detection is higher.
Evaluating the accuracy of the test
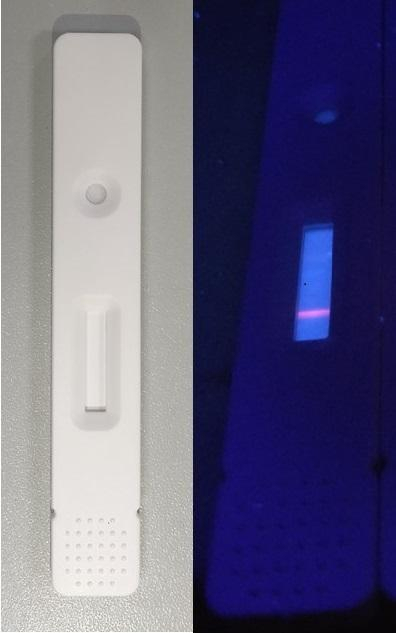
In order to evaluate the accuracy of the test, the researchers compared the test results against those of the most common COVID-19 testing technique, reverse transcriptase-polymerase chain reaction (RT-PCR). So how did they do that? Serum samples from seven novel coronavirus infection patients and 12 patients who tested negative in the RT-PCR test, but had suspicious symptoms were tested using the new LFA test.
Accurate and rapid
Just as the researchers had hoped for, the test accurately found that the seven patients were indeed infected with COVID-19. Adding further to the reliability of the test, one of the twelve suspicious cases, that had tested negative for the disease in the RT-PCR test, tested positive in the new LFA method. The other 11 suspicious cases remained negative for the coronavirus. All it took was only 10 minutes per sample.
A test with several potential applications

In a pandemic that has managed to explode globally within a few months, time is the most essential factor. A test that can diagnose the infection status of suspected cases in 10 minutes can help in timely therapeutic and social responses to help the patients and others around them. "At present, prevention of cross-infection is very essential in outbreak control, timely isolation and treatment should be adopted for this case," the authors wrote.
Another application that the researchers suggest is the use of the test to monitor the development of the disease and the effectiveness of the treatment being administered. "Our assay can be used to monitor the progression of COVID-19 as well as responses to treatment," they penned highlighting another potential use for the test.
Additionally, the immunoassay that relies on the anti-SARS-CoV-2 IgG present in the human serum, can also fulfil two other vital purposes. First is the mitigation of false negatives that RT-PCR has been criticized for. The second is the identification of potential convalescent plasma donors among recovered individuals with elevated levels of antibodies. "This assay can achieve rapid and sensitive detection of anti-SARS-CoV-2 IgG in human serum and allow positive identification in suspicious cases," concluded the researchers.









