For people who have no option but to wear contact lenses on a regular basis, the struggle to insert and remove the optical aid is a painful one. And for wearers of advancing age and with deteriorating motor functions, the danger of injury to the eyes is compounded. However, Miami man's invention could be the solution to these woes—a voice-activated robot to insert and remove contact lenses.
The inventor of the novel robot, Craig Hershoff, suffers from a condition known as Fuchs Dystrophy that requires him to wear a special lens known as scleral lenses. As an elderly individual himself, Hershoff became cognizant of the reality that several people were facing hardship due to age and dexterity while using the ocular prosthetic devices. Thus, he invented the 'Cliara Lens Robot'.

Born Out of Necessity
According to Hershoff's professional social networking website profile, he was diagnosed with Fuchs Dystrophy—a disease that affects the cornea of the eyes—in 2000. Along with three corneal transplants, he was required to wear scleral lenses for the restoration of perfect vision. It was the experience of inserting and removing these lenses that led him to build the robot, he told WPLG Local 10.
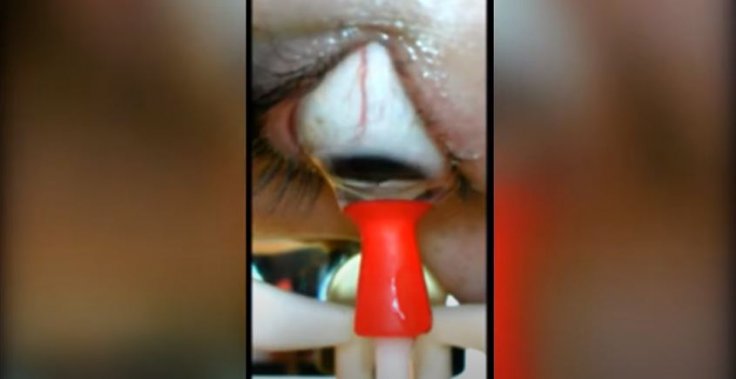
The voice-activated robot is designed specifically for those individuals whose dexterity is impeded by conditions such as arthritis and tremors caused by other ailments. Applying a safe and optimum amount of suction force for quick release and removal, the device can both insert and remove lenses. It consists of an imaging system that enables the user to monitor the removal of the lenses in real-time, giving the user complete control over the motion of the lenses.
Hopes for FDA Approval
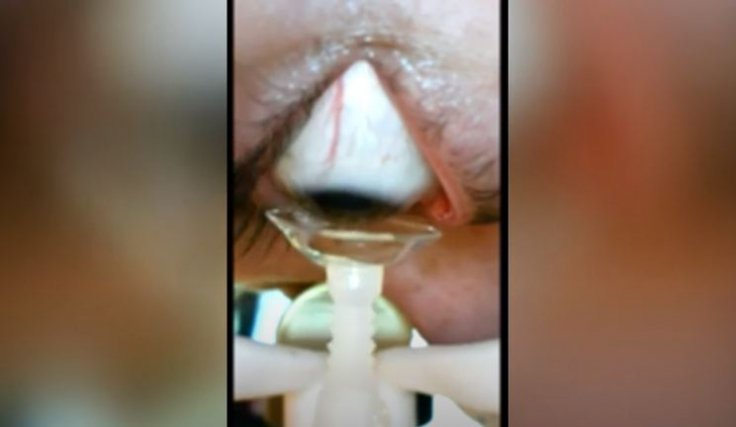
So far, the sailing has been smooth and promising for the robot. "We've tried the device on elderly people, I'm elderly too, and it really helps with dexterity. They've all liked it and appreciate how well it works," Hersoff told WPLG Local 10.
Currently, the Cliara Lens Robot is undergoing testing in a clinical trial in Boston, Massachusetts. Expressing optimism over the result of the trial, Hershoff said that he aims to receive FDA clearance for the device in early 2021.








