Of all neurogenerative diseases, Alzheimer's disease is one of the worst. It leads to the onset of dementia—an irreversible decline in memory, thinking and ability to perform simple everyday tasks—in around 60 to 70 percent of patients. Sadly, its causes continue to remain poorly understood. However, a new study has discovered that a specific mutation in a common enzyme could be the cause of the disease.
According to scientists from Tokyo Metropolitan University, a mutation in the enzyme known as MARK4 changes the properties of tau—a protein found in neurons—and increases their likeliness of aggregating and becoming insoluble. These clumps in the brain lead to the death of brain cells and lead to Alzheimer's disease.
"Here, we report that MARK4 increases the abundance of highly phosphorylated, insoluble tau species, and exacerbates neurodegeneration," wrote the authors in the study published in the Journal of Biological Chemistry.
Crucial Role Protein Clumping In Alzheimer's

Alzheimer's disease is a progressive and irreversible neurological disorder. The debilitating condition is believed to be caused when twisted clusters of tau proteins accumulate within brain cells. These viscous masses lead to the death of neurons, which in turn causes impairment of motor and memory functions.
Unfortunately, the reason behind the accumulation of tau clumps in the neurons of patients with Alzheimer's disease is not clearly understood. Therefore, any new knowledge about the mechanism and causes of the aggregation of these tau protein clumps can aid in the devising treatments and prevention of the disease.
Under normal conditions, tau proteins play a crucial role in the maintenance of the structure of neurons or the cytoskeleton. In order to keep the branches of the cytoskeleton, also known as microtubules, to be building and disintegrating constantly, the MARK4 (Microtubule Affinity Regulating Kinase 4) enzyme is said to help in the process. However, things go haywire when the gene that regulates the design for the production of MARK4 undergoes mutations.
Lethal Mutations In A Common Enzyme
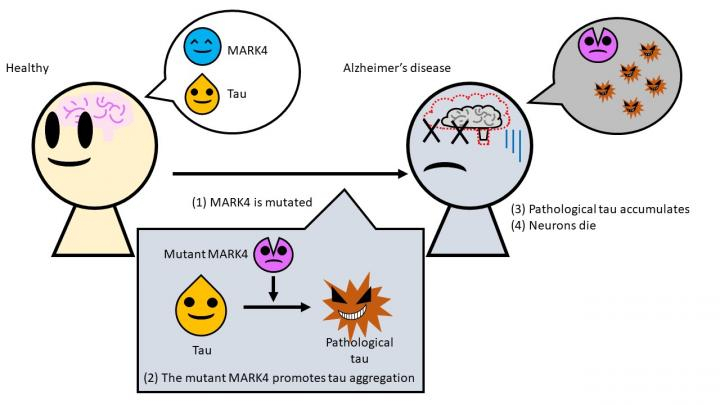
In order to find answers to the above-mentioned questions, Prof. Kanae Ando, lead author of the study, investigated the role of the MARK4 enzyme in the disease. His team introduced mutations artificially into transgenic Drosophila fruit flies—that can produce human tau proteins—and analyzed the changes in the proteins in vivo. The scientist made an interesting discovery. They found that the mutated form of MARK4 leads to changes within the tau protein, which leads to the creation of a diseased variation of tau.
Also, the 'bad' tau contains a surplus of certain chemicals that lead to its misfolding. It was also found that the bad taus aggregated with more ease and were not soluble in detergents any longer. This paved the way for the formation of the mangled clumps that lead to the degeneration of neurons.
MARK4 has been associated with a large number of diseases that involve the accumulation and clumping of several proteins. Therefore, the findings of the study may help in the development of new therapies for the treatment and prevention of a wide range of neurodegenerative disorders.









