While advancement in medical sciences over the years has led to the discovery of effective treatments for several life-threatening diseases, the cure to some still remain elusive. The diseases caused in immunocompromised individuals by the notorious hard-to-treat fungal species— Aspergillus fumigatus — is one such example. However, a new study claims that the structure of a key enzyme that makes this fungus invulnerable has been discovered.
According to a study led by Dr Lynne Howell from The Hospital for Sick Children and Dr Don Sheppard from McGill University, the structure of an enzyme known as Agd3, which plays a critical role in the formation of a protective layer known as "biofilm" around A. fumigatus, has been solved. "With structural studies, we were able to visualize where and how the enzyme binds the carbohydrate polymers and modifies them to help form the biofilm," said Howell.
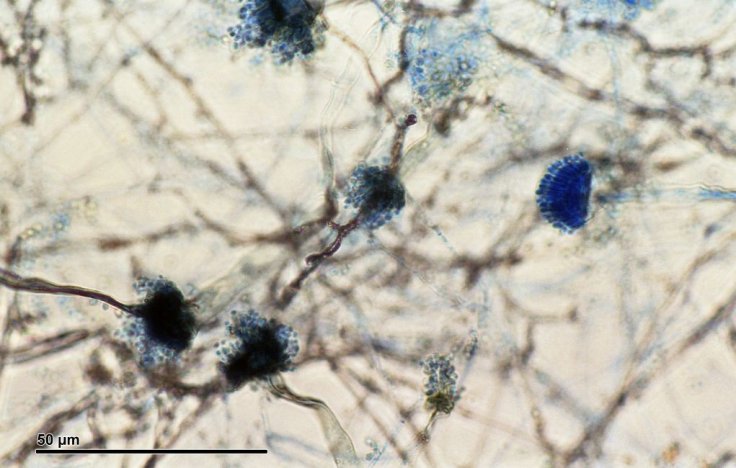
Causing Diseases In Individuals With Immunodeficiency
Aspergillus fumigates, in general, cause diseases among individuals who have compromised immune systems or immunodeficiency disorders. However, A. fumigatus, can be specifically lethal to individuals who are undergoing cancer chemotherapy or organ transplantation.
What makes the infections caused by A. fumigatus difficult to treat is the aggregation of the fungus in small communities known as "biofilms", during the infection. Not only do the biofilms provide protection from antifungal reagents, but they also assist the fungus in evading the immune system.
The enzyme, Agd3, plays a key role in the process of biofilm formation. Studies aimed at understanding this process have been ongoing as breakthroughs will offer potential therapeutic options against the virus.

'Glue' Holding The Biofilm Together
The two researchers have been collaborating for over six years to identify vulnerabilities within the biofilms produced by various pathogens. Their focus has been specifically on understanding a class of carbohydrate polymers within the pathogens that are produced by several different enzymes. The "glue" which holds the biofilm together is formed by these carbohydrate polymers.
"We want to know how these carbohydrates are synthesized and which enzymes are making them. If we know how it (biofilm) is made, we know how to take it apart," explained Sheppard
The Role of Agd3 enzyme
During the course of their study, the scientists discovered that when the Agd3 enzyme was absent, the formation of biofilm was impaired. This weakened the fungus. The duo studied in detail the 3D structure of the enzyme following the locating of the enzyme on the genome of the fungus. This enabled them in understanding the mechanism through which Agd3 aided in the formation of biofilm. It was also found that Agd3 consisted of several domains.
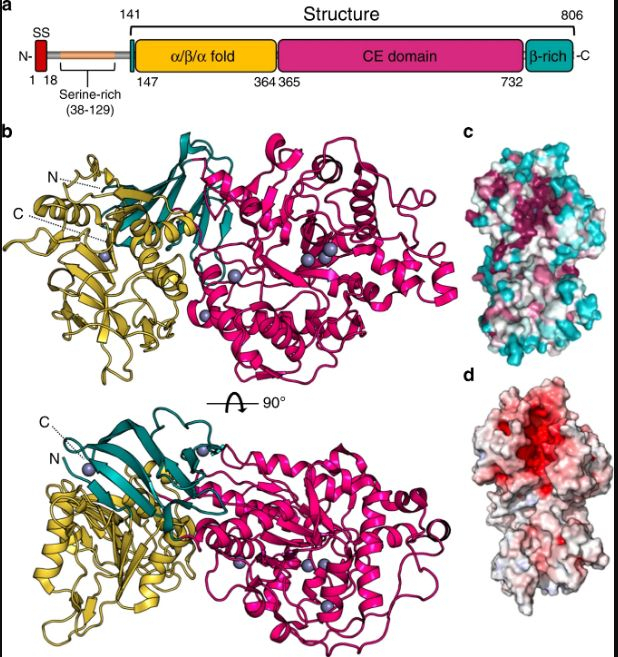
Domains are sections of protein sequences within the enzyme that can function, evolve, and exist independently from the remainder of a protein chain. Howell added that the analysis of the enzyme's structure led to the definition of a new family of carbohydrate-processing enzymes that had not been characterized previously.
Developing Treatment Options
One of the biggest global health concerns is the resistance of A. fumigatus to antifungal medications. According to Sheppard, deeper insights into the structure of Agd3 facilitate in the development of treatment strategies.
As the next step, the team wishes to use the data gathered from the structural analysis of Agd3 and how antibodies bind to it, in order to formulate antibody therapeutics to treat infections by A. fumigates. "We are now designing antibodies that can inhibit the function of Agd3 based on structural information we gathered," concluded Sheppard.









