Singapore's Health Sciences Authority (HSA) has warned consumers about banned substances that were found in two popular weight-loss products. Consumers of the two slimming products -- Serifa Beauty Solidmolid and LKS Coffee -- have experienced sudden weight loss and loss of appetite while some have reported rapid heartbeat, it said.
The substance in question, sibutramine, has been banned in Singapore since 2010 due to its adverse effects and increased risks of heart attacks and strokes. When it was available, it used to be a prescription drug for weight loss. But it was banned following reports of insomnia, visual and auditory hallucinations as adverse effects.
False Claims
Both products are in wide circulation over e-commerce and social media platforms with many influencers advocating for both. However, HSA has already notified the sellers to take down the listings. "Warnings have been issued to the sellers and the respective website administrators have been directed to take down the affected listings," the HSA said in a statement on Monday, June 29.


The Serifa Beauty Solidmolid is targeted to consumers who are seeking to lose weight with claims of "burning fats up to 10X". The LKS too claimed to "accelerate fat burning" and "promote metabolism" to "achieve the perfect slimming effect." The HSA has advised the consumers to stop taking both immediately and seek medical attention if they have any adverse side effects.
"All sellers and suppliers must stop selling these products immediately. It is illegal to sell and supply products containing banned substances. Sellers and suppliers are liable to prosecution and if convicted, may be imprisoned for up to two years and/or fined up to $10,000," HSA said.
Earlier this month, three more weight-loss products were banned by HSA for similar reasons. Clinic K and RO Slim Booster were found to contain sibutramine while the other, Rozell Detox, had another banned substance Sennoside. Consumers of those products reportedly experienced rapid heartbeat, breathlessness and dizziness within two days of taking the product. HSA also found that both Rozell Detox and RO Slim Booster were marketed as natural products but were not.


Standardised Packaging Must for Tobacco Products
In another development, the Singapore Ministry of Health said all tobacco products must have standardized packaging and enlarged graphic health warnings from July 1. In an effort to encourage smokers to quit, MOH said that all tobacco products including cigarettes, cigars, beedies, cigarillos, ang hoon besides roll-your-own tobacco products must contain the warnings.
The tobacco manufacturing companies must remove all brand logos, images and any promotional material from the packaging. Instead, they will have to display the brand name and variant on the pack in a standard color and font with graphic health warning covering 75 percent of the package.
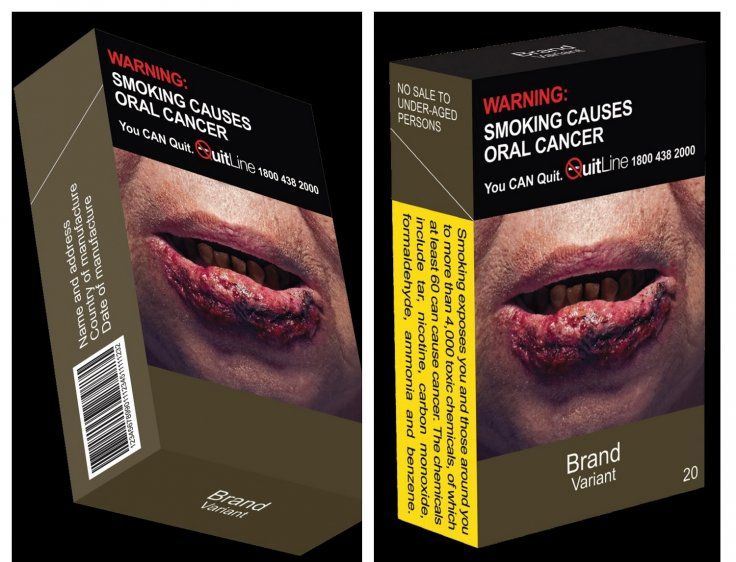
"The SP Measure for tobacco products is part of a multi-pronged approach aimed, among others, at discouraging non-smokers from picking up smoking, encouraging smokers to quit, and encouraging Singaporeans to adopt a tobacco-free lifestyle, which will ultimately lead to reduced smoking prevalence," the MOH said in a press release.
MOH had allowed a 12-month transition period to tobacco manufacturers, importers, wholesalers and retailers to implement the measures. Non-compliance with the regulation will attract S$10,000 fine and/or imprisonment up to six months. Repeated offenders will face heavier penalties.









