Recently, Tamiflu side effects causing neuropsychiatric events, especially in children, have been creating headlines. Stories about the frightening side effects are not new and have been doing the rounds since 2009. People in America are now demanding better labeling on the packaging and warnings from doctors before prescribing the shot.
More families are now coming up with their stories. CBS11 first broke the news of a six-year-old girl who tried jumping out of her window after taking the influenza shot, followed by 11-year-old Lindsay Ellis from Indianapolis who hallucinated bugs on her body. Even worse, she apparently heard the devil's voice in her head. For her father Charles Ellis, the scenario was similar to a horror movie and he even thought that his daughter might be possessed.
Ellis started acting strangely on the third day after taking the flu shot. She had to be hospitalized for two months and had a feeding tube attached as she was unable to move her hands or feet. Ellis still gets tremors.
About Tamiflu
Tamiflu is an anti-viral medication that deals with the influenza virus and should be taken within 48 hours of the onset of the symptoms. It cannot cure the flu but can help reduce the duration of the symptoms. Children with lung diseases, cancer, heart diseases, neuromuscular disorders are considered at high risk.
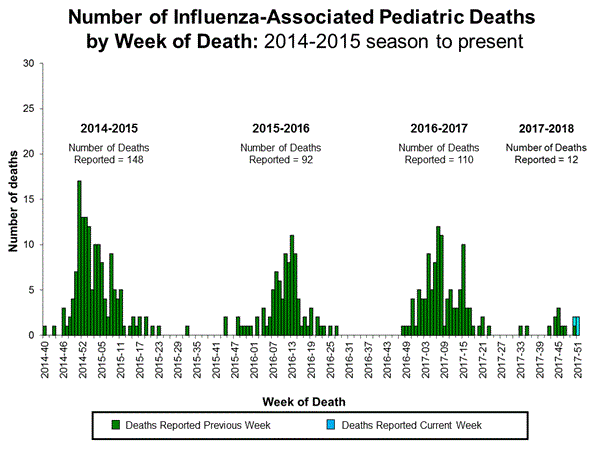
Japan's take on Tamiflu
Japan, in 2007, banned Tamiflu for youths aged 10 to 19 after cases of neuropsychiatric events. A Genentech spokesperson told CBS11 in a statement that neuropsychiatric events attack during the administration of Tamiflu in patients suffering from influenza. However, these side effects also occur in patients suffering from influenza but who have not taken Tamiflu. There have been 559 hallucination cases since 2009.
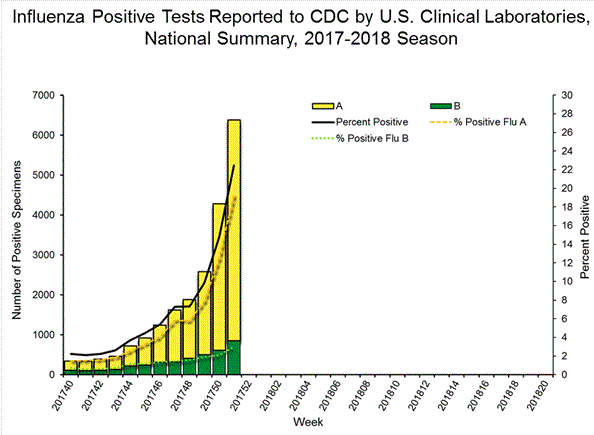
WHO's take on Tamiflu
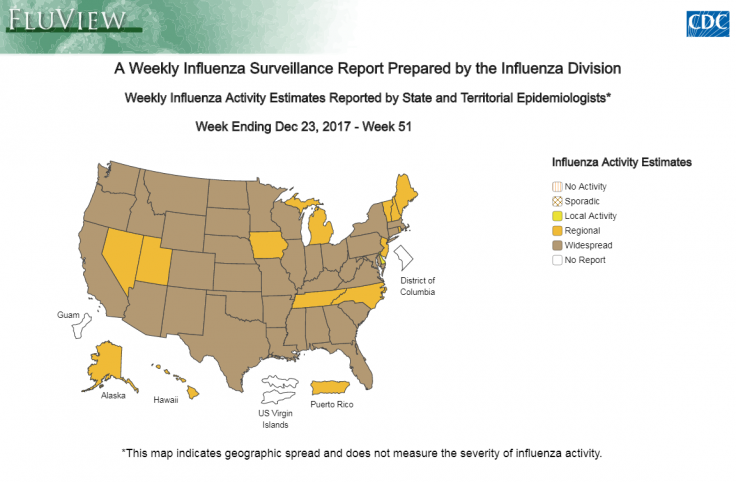
The World Health Organization (WHO) downgraded the influenza shot on its essential medicine list. Tamiflu was approved by the US Food and Drug Administration (FDA) in 1999 and the European Medicines Agency (EMA) in 2002 for the treatment of influenza within 48 hours. This information, however, came from limited data based on random trials.

After the outbreak of avian influenza and H1N1 pandemic in 2009, governments around the world stockpiled Tamiflu and the shot generated over $18bn in sales worldwide, half of it coming from government stockpile. In spite of these, the FDA concluded that there is no evidence that proves that Tamiflu reduces influenza symptoms, complications or mortality.








