With Pfizer and Moderna's COVID-19 vaccines showing promising results in clinical trials, the world has a sigh of relief. Combined with other approved vaccines, it can potentially end the Coronavirus pandemic that has already infected over 55 million people and killed over 1.3 million worldwide. However, both vaccines potentially pose a logistical nightmare as they are to be kept cold.
Moderna's COVID-19 vaccine can be kept at -20° Celsius or -4° Fahrenheit. Thus, a regular deep freezer could do the job. but the vaccine from Pfizer-BioNTech poses a greater challenge. It must be kept at -70° Celsius or -94° Fahrenheit to be effective. The temperature needed for the Pfizer vaccine is colder than winter in Antarctica thus conventional cold storage system won't be effective.

Why Does It Need Such Cold Temperature?
The U.S. vaccine makers suggested that both candidates were found to be over 90 percent effective in staving off COVID-19 infection. But why does the Pfizer vaccine need to be kept at such a low temperature? The answer is in the vaccine architecture. Both Pfizer and Moderna used mRNAs (messenger Ribonucleic acid) that introduce DNA instructions and a template to build a protein to trigger the body's immune response against COVID-19.
The mRNA technique is new and the vaccines are cheaper and faster to produce. Once approved, either of Moderna and Pfizer whichever comes first will be the first mRNA-based vaccine to get U.S. Food and Drug Administration's (FDA) nod.
However, there is a problem with the technique. Margaret Liu, a vaccine researcher from the International Society for Vaccines, told NPR that mRNA could be "really easily destroyed, and that's because there are many, many enzymes that will just break it apart."
That's because the mRNA nucleosides were modified for the vaccine and then using lipid nanoparticles to keep it intact, Liu explained. However, if it's not frozen, the lipid coating and mRNA can crumble. That's the reason it needs to be frozen at such a low temperature.
"Everything happens more slowly as you lower the temperature. So, your chemical reactions — the enzymes that break down RNA — are going to happen more slowly," Liu said, adding that Moderna's lipid coating might be more stable and that's the reason it would not need ultra-low temperature like Pfizer's.
Logistical Nightmare
With the good news of being over 90 percent effective, comes the bad news. It will need special logistical arrangements to transport the vaccines to hospitals.
Pfizer said that it would use special boxes with specially formulated dry ice (solid carbon dioxide) that could keep 1,000 to 5,000 doses of vaccines each. But it can keep the vaccines for only 10 days and the dry ice must be replaced every five days for up to 15 days maximum before it is placed in ultra-freezers. The other problem is that the special box can be opened only twice a day for less than three minutes.

Hence, as Pfizer starts shipping from its two facilities in Kalamazoo, Michigan and Pleasant Prairie, Wisconsin, it will take around four to five days for the vaccines to arrive at the hospitals which will then have only five to six days left to administer the doses if they don't have special freezers. So, the hospitals may need to administer around 833 doses a day. But that also poses a challenge as the boxes can be opened only twice a day. Furthermore, Pfizer and Moderna both vaccines need two doses, once in 21 days.
For hospitals to arrange for special freezers, it will likely to incur huge costs. Each ultra-cold freezer costs around $20,000 each and they are not readily available to buy. One of the manufacturers in the U.S., K2 told CBS MoneyWatch that such freezers would take at least six weeks to be delivered.
"The reality is there has never been a drug that required storage at this temperature. The administration and distribution effort will require an all-hands-on-deck," Soumi Saha, a pharmacist and director Premier, an advocacy group, told CBS News.
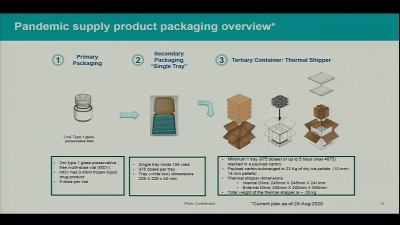

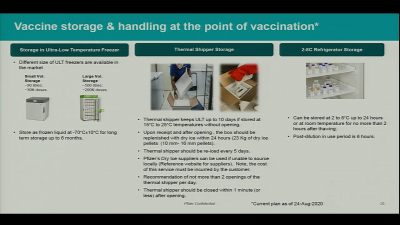
Existing Cold Storage Facilities Not Enough
One potential solution could have been to store vaccines at existing cold storage warehouses before distributing them to hospitals. But the current cold storage facilities are already overwhelmed and have only five percent space available. In the Philippines, authorities suggested partnering ice cream manufacturers to store Coronavirus vaccines.
However, the situation is different in the U.S. The government said that it would cover the cost of vaccines. But there is no clarity on whether the government will also provide the fund to hospitals for the storage. Some hospitals already have special freezers but those are mainly used for laboratory purposes and would need permission from local health inspectors to repurpose the freezers for vaccine storage.
Ebola vaccines were kept at -80 in DRC. Here's my photo from Beni. They go from the big freezers, to the green Arktek canisters, to dry ice canisters in freezer boxes that are transported on motorbike down dirt roads.
— Amy Maxmen (@amymaxmen) November 12, 2020
Where there's $$$, political will and a plan, there's a way. https://t.co/5mA7mVFUuY pic.twitter.com/UTmOMgYx1L
In the rural areas of the U.S., the situation is even worse. Hospitals there don't have such facilities to store vaccines. They don't have enough staff nor the population to administer around 1,000 doses a day, making the effort not feasible, reported Slate.
As for other countries including Chile, Australia, Japan, the U.K., and Canada besides European Union nations, the special freezer is expected to pose a tough challenge as well. Until Pfizer can stable the vaccine at a nominal temperature and lower the cost further (it will cost around $20), it will not be able to market it in smaller European nations, South America and Asian countries like India.









