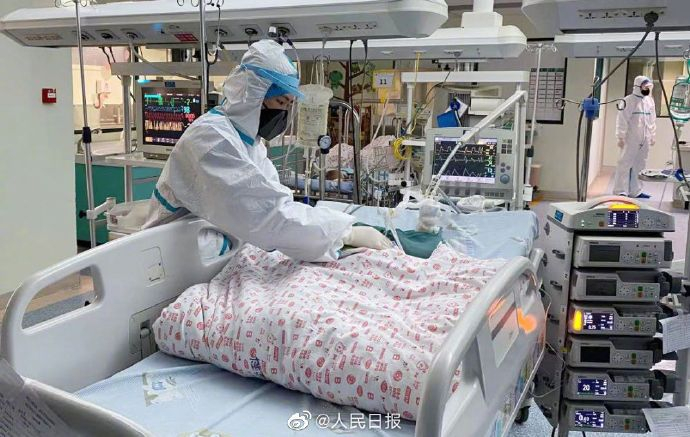
As per the recent official update, novel coronavirus has now infected over 67,000 people globally and the death toll is at least 1,526, including three people outside mainland China. While there are several claims about the cure of this virus outbreak, new reports revealed that blood from cured coronavirus patients could help to treat infected individuals.
A Chinese senior health official asked recovered patients to donate blood plasma on Thursday, February 13, which could be useful to treat sick patients.
The Blood plasma donation
It should be noted that the donation call came after China National Biotec Group, a state-owned company announced that these antibodies helped treat 10 critically ill coronavirus patients by reducing the inflammation within 12 to 24 hours, reported The New York Times.
As per the experts, even though this newly introduced approach is a logical and promising way to treat critically ill coronavirus patients, doctors should be on high alert for possible side effects. The medical experts also mentioned that since the coronavirus has a low mortality rate, bypassing the normal drug testing procedure doesn't necessarily make sense.

How the antibodies can help
It should be mentioned that the antibodies are proteins which the immune system creates to combat viruses, bacteria or other foreign substances. But it takes time for a body to fasten up its antibody production to fight against a new invader. If the same bacteria or virus tries to attack the body again in the future, the body will remember it and quickly produce an army of antibodies.
As per the experts, individuals who recovered from COVID-19 still have the antibodies to the coronavirus circulating in their blood. So, if those antibodies are injected into sick patients, it could help them to fight against the virus.
Experts on new coronavirus treatment approach
It should be noted that this new approach will also help to transfer the immunity of a recovered coronavirus patient to a sick person which has been used earlier during the flu pandemics, said a professor of epidemiology at the University of Hong Kong, Benjamin Cowling.
Carol Shoshkes Reiss, a professor of biology and neural science at New York University who was not part of this new research stated that the people behind the new approach need to control for possible effects of the treatment.
Dr Eric Cioe-Peña, the director of global health at Northwell Health in New York, said, "I think these theoretic[al] treatments are good ideas, but nothing about this virus or these infections makes me want to skip the normal process we use to make sure that a treatment is safe and effective before subjecting people to it."
In addition, Dr Eric believes that the scientific process should be allowed to continue and "Attempt to study these proposed treatments before enacting them, especially in a virus that has such a low mortality."
Recent treatment claims
Earlier a Pennsylvania-based American biotech company, Inovio Pharmaceuticals, claimed that they created a vaccine for the coronavirus three hours after they got access to the virus' genetic sequence in the mid-January.
As reported by FOX Business, Dr J Joseph Kim, Inovio's president said, "We were able to rapidly construct our vaccine in a matter of about three hours once we had the DNA sequence from the virus available because of the power of our DNA medicine platform. Our goal is to start phase one human testing in the U.S. early this summer."









